Abstract
Introduction
Clostridium difficile infection (CDI) is a major cause of morbidity and mortality in hospitalized patients. In autologous stem cell transplant (ASCT) recipients the national estimated incidence is approximately 5% to 10%. The use of antibacterial agents, such as ciprofloxacin, which historically has been used as a prophylactic agent in our autologous stem cell transplant population, is considered to be a risk factor for developing CDI. However, there have been few studies that have investigated the relationship of prophylactic antibiotics in the ASCT population and the development of CDI. One study, Satlin et al, found fluoroquinolone prophylaxis in ASCT patients decreased the rate of febrile neutropenia and blood stream infections and also a possible increase in the rate of CDI.
Methods
To attempt to decrease the incidence of CDI, our institution made a change to our standard of practice and suspended the utilization of CIP (500 mg twice daily beginning on the day zero and continued until engraftment or antibiotic escalation) for antibacterial prophylaxis in our ASCT population. All patients received antiviral and antifungal prophylaxis at baseline and broad-spectrum anti-pseudomonal antibacterial agents at the first recorded febrile event or as clinically necessary. CDI testing was completed when patients had three or more liquid stools in a 24-hour period along with other symptoms (i.e., fever, abdominal pain). Patients were found to have a CDI when the stool sample resulted positive while experiencing correlative symptoms. Stool samples were assessed for Clostridium difficile with the Cepheid GeneXpert NAAT. We retrospectively collected data on patients who received CIP prophylaxis from June 2016 - June 2017 and compared it to patients who did not receive CIP prophylaxis from July 2017 - June 2018. Data were analyzed using Chi-squared and T-tests were used to assess for significance and logistic regression was utilized to assess for possible associations between patient characteristics (age, race, sex, diagnosis, and conditioning regimen) and CDI.
Results
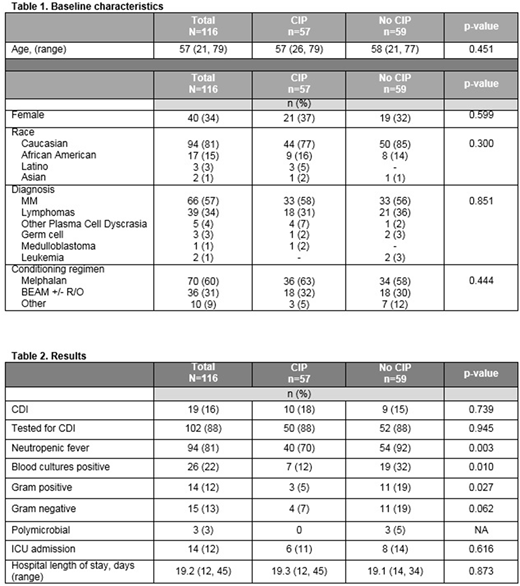
A total of 116 ASCT patients were analyzed; the median age was 57 years old and the most common diagnoses were multiple myeloma, non-Hodgkin's lymphoma, and Hodgkin's lymphoma; 57 patients received CIP antibacterial prophylaxis and 59 patients received no antibacterial prophylaxis. The most common conditioning regimens include melphalan (60%) and BEAM with or without rituximab (22%). CDI occurred in 15% of patients who did not receive CIP prophylaxis and 18% of patients who received prophylaxis (p=0.739). Of note, 88% of patients in both groups were tested for CDI. Neutropenic fever occurred significantly more often in patients who did not receive prophylaxis, 64% vs 35% (p=0.003). The rates of bacteremia were also found to be significantly higher in the non-CIP group, 32% vs. 12% (p=0.01). Gram-negative bacteria blood isolates were increased in patients not receiving CIP prophylaxis, 19% vs. 7% (p=0.062). Interestingly, there was also an increase in the occurrence of gram-positive bacteremia isolates, 19% vs. 5% (p=0.03). The average length of hospital stay was 19 days for both groups and intensive care unit admissions were similar between the two groups, 14% vs. 11% (p=0.616).
Conclusion
The lack of CIP prophylaxis in ASCT did not significantly decrease the rate of CDIs. With the removal of antibacterial prophylaxis, there was a significant increase in the rate of neutropenic fever and bacteremia, specifically gram-positive bacteremia. Hospital length of stay and admissions to the intensive care unit were similar between the groups. No additional risk factors were determined to be associated with the development in CDI in the patient population. Based on the results of this study, our institution reinstituted the utilization of CIP prophylaxis. Future studies include the utilization of antibody prophylaxis for CDI and also assessing Clostridium difficile colonization and its effect on developing an active clinical infection.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal